클로메스트로네
Clomestrone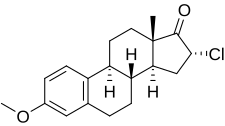 | |
| 임상 데이터 | |
|---|---|
| 상호 | 아테롤로, 아테란, 콜레스트렐, 이포스클레론, 리프로텐, 페르스클레롤 |
| 기타 이름 | SC-8246; 16α-클로로에스트론 3-메틸에테르; 16α-클로로-3-메톡시에스트라-1, 3, 5(10)-트리엔-17-온 |
| 루트 행정부. | 입으로 |
| 약물 클래스 | 에스트로겐, 에스트로겐 에테르 |
| 식별자 | |
| |
| CAS 번호 | |
| PubChem CID | |
| 켐스파이더 | |
| 유니 | |
| CompTox 대시보드 (EPA ) | |
| ECHA 정보 카드 | 100.021.669 |
| 화학 및 물리 데이터 | |
| 공식 | C19H23클론O2 |
| 몰 질량 | 318.84 g/g−1/g/g/sqm |
| 3D 모델(JSmol) | |
| |
| |
클로메스트론(상표명 Arterolo, Atheran, Colestrel, Iposclerone, Liproten, Persclerol, 기타), 또는 16α-클로로에스트론 3-메틸에테르라고도 알려진 SC-8246은 에스트론에서 유래한 합성 스테로이드성 약 에스트로겐으로, 항에스테르 처리제로 사용됩니다.임상연구에서 대부분의 환자에게서 유방 압통,[3][4] 성욕 상실, 피로 또는 탈락을 포함한 일부 에스트로겐성 부작용이 관찰되었지만, 그것은 최소한의 여성화를 생성하면서 혈청 지질 프로파일에 이로운 영향을 미친다고 한다.이 약은 미타트리엔디올과 유사하며 두 에스트로겐은 유사한 약물 프로파일을 [5]가지고 있다.클로메스트론은 1958년 문헌에 기술되었고 그 [1]직후 의료용으로 도입되었다.
「 」를 참조해 주세요.
레퍼런스
- ^ a b J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 297–298. ISBN 978-1-4757-2085-3.
- ^ Gregory Pincus (22 October 2013). Hormones and Atherosclerosis: Proceedings of the Conference Held in Brighton, Utah, March 11-14, 1958. Elsevier Science. pp. 253–374. ISBN 978-1-4832-7064-7.
- ^ RIVIN AU (1959). "SC 8246, a new estrogen analog: lipoprotein effects with minimal feminization". Metab. Clin. Exp. 8: 704–8. PMID 14437693.
SC-8246 (16-alpha chlorestrone 3-methyl ether) was administered for periods of six to twelve months to 20 male survivors of acute myocardial infarction ranging in age from 30 to 63 years. A significant decrease in serum cholesterol concentration occurred in only 6 of 13 patients with an initial cholesterol level above 250 mg. per 100 ml., and there was no change in the other 7. Of 7 initial cholesterol levels below 250 mg. per 100 ml., no level decreased, 3 increased, and 4 were unchanged. In 9 of 11 patients with an initial alpha:beta lipoprotein ratio of less than 20 per cent, a significant increase occurred, but no change in the other 2. Among 9 subjects with a ratio initially above 20 per cent, a further increase occurred in 8 while taking the drug. This estrogen appeared to have an advantage in terms of lessening side-effects. Mild breast tenderness or gynecomastia occurred in 15 of the 17 patients with a "favorable" lipoprotein change. When the dosage was reduced to 5 mg. daily or every other day, the lipoprotein effect in 8 of them could be sustained while the breast changes disappeared. Libido disappeared from 2 patients and was diminished in 1 other. Other side-effects were nausea in 1 patient, loss of ambition in 5, and itching or dryness of the skin in 4.
- ^ WINSOR T, FISHER EK, PAYNE JH (1959). "A method for the study of peripheral arteriosclerosis". J Am Geriatr Soc. 7 (2): 167–74. doi:10.1111/j.1532-5415.1959.tb01062.x. PMID 13630690. S2CID 46048052.
- ^ Cancer Chemotherapy Abstracts. Information Resources Press. January 1960. p. 143.


