피토카나비노이드 비교
Comparison of phytocannabinoids칸나비노이드(/kˈnébənɔdzˌæ knnəbɔn/dz/)는 칸나비스 식물 또는 [1][2]엔도카나비노이드 시스템과 상호작용할 수 있는 합성 화합물이다.가장 주목할 만한 칸나비노이드는 대마초 [3][4]내 1차 중독 화합물인 피토카나비노이드 테트라히드로카나비놀(THC)이다.칸나비디올([5]CBD)은 일부 대마초의 또 다른 주요 성분이다.적어도 113개의 다른 대마초들이 [6]대마초에서 분리되었다.
이 기사는 보다 일반적인 자연 및 합성 카나비노이드 중 일부에 대한 비교 구조를 제공할 뿐만 아니라 법적으로 금지되고 허가된 카나비노이드 구조를 보여준다.
구조물들
| 칸나비노이드 | 2D 구조 | 3D 구조 |
|---|---|---|
| CBC |  |  |
| CBCV |  |  |
| CBD | 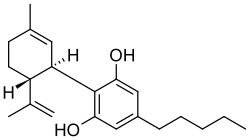 | 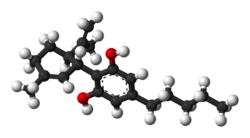 |
| CBDP |  |  |
| CBDV |  |  |
| CBE |  |  |
| CBG |  |  |
| CBGV |  |  |
| CBL |  |  |
| CBN | 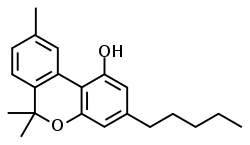 | 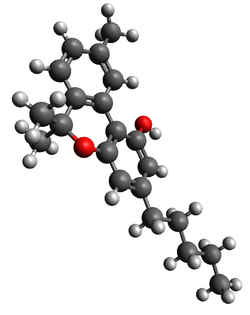 |
| 중앙 |  |  |
| CBTC |  |  |
| CBV |  |  |
| 델타-8-THC |  |  |
| THC |  | 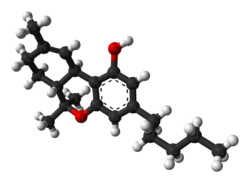 |
| THCC |  |  |
| 챠흐 |  |  |
| THCP |  |  |
| THCV |  |  |
합법성
| 칸나비노이드 | 법적 상태 |
|---|---|
| CBCA, CBC | 대부분의 국가에서 합법적 |
| CBCVA, CBCV | |
| CBDA, CBD | |
| CBDPA, CBDP | |
| CBDVA, CBDV | |
| CBEA, CBE | |
| CBGA, CBG | |
| CBGAM, CBGM | |
| CBGVA, CBGV | |
| CBLA, CBL | |
| CBNA, CBN | |
| CBNDA, CBND | |
| CBTA, CBT | |
| CBVA, CBV | |
| THCA, THC | 향정신성 물질에 관한 유엔 협약 |
| THCCA, THCC | |
| 챠흐 | 대부분의 국가에서 합법적 |
| THCPA, THCP | 대부분의 국가에서 합법적 |
| THCVA, THCV |
열특성
변환 온도
| 가열 카나비노이드 | 변환 온도 | 결과적으로 생기는 칸나비노이드 |
|---|---|---|
| CBC | 250°C(482°F)~300°C(572°F)[7] | THC |
탈탄산화 온도
가열 시, 칸나비노이드산은 탈탄산염되어 정신 활성 칸나비노이드를 생성한다.예를 들어, Delta-9-tetrahydrocannabinol(THC)은 대마초에서 발견되는 주요 정신 활성 화합물이며 섭취 시 "높은" 느낌을 일으킨다.그러나 대마초에는 자연적으로 상당한 양의 THC가 포함되어 있지 않다.대신 테트라히드로카나비놀산(THCA)은 생 및 살아있는 대마초에서 자연적으로 발견되며 독성이 없다.시간이 지남에 따라 THCA는 약 1년에 걸쳐 탈탄산화 과정을 통해 서서히 THC로 전환되지만 고온에 노출되면 속도가 빨라질 수 있습니다.110°C의 조건에서 가열할 경우 탈탄산화는 일반적으로 30-45분 후에 발생합니다.이것은 대마초에 첨가된다.경구 섭취 시 간은 분해되어 보다 강력한 11-히드록시-THC로 대사된다.
여기에 나열된 모든 칸나비노이드와 그 산은 식물에서 자연적으로 다양한 정도로 발견됩니다.
| 탈탄산화 반응 | 온도 |
|---|---|
| CBCA → CBC | |
| CBCVA → CBCV | |
| CBDA → CBD | |
| CBDPA → CBDP | |
| CBDVA → CBDV | |
| CBEA → CBE | |
| CBGA → CBG | |
| CBGAM → CBGM | |
| CBGVA → CBGV | |
| CBLA → CBL | |
| CBNA → CBN | |
| CBNDA → CBND | |
| CBTA → CBT | |
| CBVA → CBV | |
| THCA → THC | 110°C(230°F)[8] |
| THCCA → THCC | |
| THCPA → THCP | |
| THCVA → THCV |
기화 온도
드라이허브 기화기는 꽃 모양의 대마초를 흡입하기 위해 사용될 수 있다.대마초 공장에는 483개의 식별 가능한 화학 성분이 있으며,[9] 적어도 85개의 다른 대마초가 식물에서 분리되었다.방향족 테르페노이드는 126.0°C(258.8°F)에서 증발하기 시작하지만, 생물 활성성이 더 높은 테트라히드로카나비놀(THC)과 칸나비올(CBD), 칸나비크롬(Cannabichrom)과 같은 칸나비노이드(cannabis)에서 발견되는 다른 칸나비노이드도 있다.ts를 클릭합니다.
여기에 나열된 칸나비노이드는 식물에서 발견되지만 미량에서만 발견됩니다.그러나 온라인에서는 분리주로서도 추출되어 판매되고 있습니다.서드파티 인증은 구매자가 합성 카나비노이드를 사용하지 않도록 하는 데 도움이 될 수 있습니다.
| 칸나비노이드 | 비등점 |
|---|---|
| CBC | 220°C(428°F)[10] |
| CBCV | |
| CBD | 160°C(320°F)-180°C(356°F)[10] |
| CBDP | |
| CBDV | |
| CBE | |
| CBG | |
| CBGM | |
| CBGV | |
| CBL | |
| CBN | 185°C(365°F)[10] |
| CBT | |
| CBV | |
| 델타-8-THC | 175°C(347°F)-178°C(352°F)[10] |
| THC | 157°C(315°F)[10] |
| THCC | |
| THCP | |
| THCV | 220 미만[10] |
구조 스케줄링
| 칸나비게롤형(CBG) | ||||
|---|---|---|---|---|
 칸나비게롤 |  칸나비게롤 | 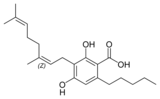 카나비네롤산 A |  칸나비게로바린 | |
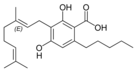 칸나비게롤산A |  칸나비게롤산A |  칸나비게로바린산A | ||
| 칸나비크로멘형(CBC) | ||||
 (±)-카나비크로멘 | 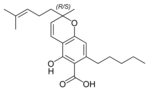 (±)-카나비크롬산 A | 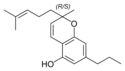 (±)-카나비바리크로멘(±)-카나비크롬바린 |  (±)-카나비크롬바린성 | |
| 칸나비디올형(CBD) | ||||
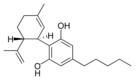 (-) 카나비디올 |  칸나비디올 | 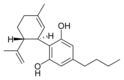 칸나비디올-C4 | 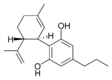 (-)카나비디바린 |  칸나비디오콜 |
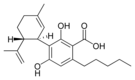 칸나비디올산 |  칸나비디바린산 | |||
| 칸나비노디올형(CBND) | ||||
 칸나비노디올 | 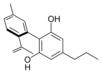 칸나비노디바린 | |||
| 테트라히드로카나비놀형(THC) | ||||
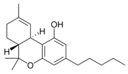 δ-테트라히드로카나비놀9 |  δ-테트라히드로카나비놀-C94 |  δ-테트라히드로카나비바린9 |  δ-테트라히드로카나비오콜9 | |
 δ-테트라히드로-9 | 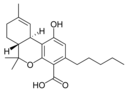 δ-테트라히드로-9 |  δ-테트라히드로-9 | 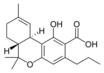 δ-테트라히드로-9 |  δ-테트라히드로-9 |
 (-)-트랜스-8(6aR, 10aR)- |  (-)-트랜스-8(6aR, 10aR)- |  (-)-(6aS, 10aR)-δ-9 | ||
| 칸나비놀형(CBN) | ||||
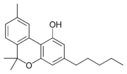 칸나비놀 |  칸나비놀-C4 |  칸나비바린 | 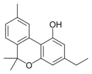 칸나비놀-C2 |  칸나비오콜 |
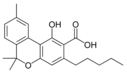 칸나비놀산 A | 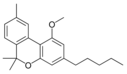 칸나비놀메틸에테르 | |||
| 칸나비트리올형(CBT) | ||||
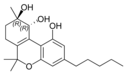 (-)-(9R, 10R)-트랜스- | 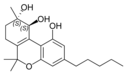 (+)-(9S, 10S)-카나비트리올 |  (±)-(9R, 10S/9S, 10R)- |  (-)-(9R, 10R)-트랜스- |  (±)-(9R, 10R/9S, 10S)- |
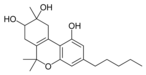 8,9-디히드록시-δ-6a(10a) |  칸나비디올산A |  (-)-(6aR, 9S, 10S, 10aR)- |  (-)-6a, 7,10a-트리히드록시- |  10-옥소-δ-6a(10a) |
| 칸나비엘소인형(CBE) | ||||
 (5aS, 6S, 9R, 9aR)- |  (5aS, 6S, 9R, 9aR)- | |||
 (5aS, 6S, 9R, 9aR)- |  (5aS, 6S, 9R, 9aR)- |  (5aS, 6S, 9R, 9aR)- | ||
 칸나비글렌돌-C3 |  데히드로카나비푸란 |  칸나비푸란 | ||
| 이소카나비노이드류 | ||||
 (-)-트랜스-7(1R, 3R, 6R)- |  (±)-δ-17, 2-cis- | 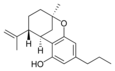 (-)-트랜스-7(1R, 3R, 6R)- | ||
| Cannabicclol 타입(CBL) | ||||
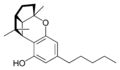 (±)-(1aS, 3aR, 8bR, 8cR)- |  (±)-(1aS, 3aR, 8bR, 8cR)- | 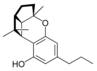 (±)-(1aS, 3aR, 8bR, 8cR)- | ||
| 칸나비크란형(CBT) | ||||
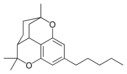 칸나비크란 | ||||
| 칸나비치로마논형(CBCN) | ||||
 칸나비치로마논 |  칸나비치로마논-C3 |  칸나비쿠마로논 | ||
레퍼런스
- ^ Abyadeh M, Gupta V, Paulo JA, Gupta V, Chitranshi N, Godinez A, et al. (September 2021). "A Proteomic View of Cellular and Molecular Effects of Cannabis". Biomolecules. 11 (10): 1411–1428. doi:10.3390/biom11101411. PMC 8533448. PMID 34680044.
- ^ "Marijuana, also called: Cannabis, Ganja, Grass, Hash, Pot, Weed". Medline Plus. 3 July 2017.
- ^ Lambert DM, Fowler CJ (August 2005). "The endocannabinoid system: drug targets, lead compounds, and potential therapeutic applications". Journal of Medicinal Chemistry. 48 (16): 5059–5087. doi:10.1021/jm058183t. PMID 16078824.
- ^ Pertwee R, ed. (2005). Cannabinoids. Springer-Verlag. p. 2. ISBN 978-3-540-22565-2.
- ^ "Bulletin on Narcotics – 1962 Issue 3 – 004". UNODC (United Nations Office of Drugs and Crime). 1962-01-01. Retrieved 2014-01-15.
- ^ Aizpurua-Olaizola O, Soydaner U, Öztürk E, Schibano D, Simsir Y, Navarro P, et al. (February 2016). "Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes". Journal of Natural Products. 79 (2): 324–331. doi:10.1021/acs.jnatprod.5b00949. PMID 26836472.
- ^ Czégény, Z; Nagy, G; Babinszki, B; Bajtel, Á; Sebestyén, Z; Kiss, T; Csupor-Löffler, B; Tóth, B; Csupor, D (26 April 2021). "CBD, a precursor of THC in e-cigarettes". Scientific Reports. 11 (1): 8951. Bibcode:2021NatSR..11.8951C. doi:10.1038/s41598-021-88389-z. PMC 8076212. PMID 33903673.
- ^ Wang, M; Wang, YH; Avula, B; Radwan, MM; Wanas, AS; van Antwerp, J; Parcher, JF; ElSohly, MA; Khan, IA (2016). "Decarboxylation Study of Acidic Cannabinoids: A Novel Approach Using Ultra-High-Performance Supercritical Fluid Chromatography/Photodiode Array-Mass Spectrometry". Cannabis and Cannabinoid Research. 1 (1): 262–271. doi:10.1089/can.2016.0020. PMC 5549281. PMID 28861498.
- ^ El-Alfy; Abir T; et al. (Jun 2010). "Antidepressant-like effect of delta-9-tetrahydrocannabinol and other cannabinoids isolated from Cannabis sativa L". Pharmacology Biochemistry and Behavior. 95 (4): 434–42. doi:10.1016/j.pbb.2010.03.004. PMC 2866040. PMID 20332000.
- ^ a b c d e f "Phytocannabinoid Boiling Points" (PDF). projectcbd.org. Archived (PDF) from the original on 2019-04-08. Retrieved 20 August 2021.
- ^ (PDF) https://leg.mt.gov/content/Committees/Interim/2009_2010/Children_Family/Emerging-Issue/mmga-presentation-cannabinoid-table-aug2010.pdf.
{{cite web}}:누락 또는 비어 있음title=(도움말)

