MN-25
MN-25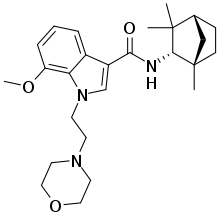 | |
| 임상 데이터 | |
|---|---|
| 다른 이름들 | N-[(S)-Fenchyl]-1-[2(morpholin-4-yl)(]-7-methoxyindole-3-carboxamide |
| 법적 상태 | |
| 법적 상태 |
|
| 식별자 | |
| |
| CAS 번호 |
|
| PubChem CID | |
| 켐스파이더 | |
| 유니 | |
| ChEMBL | |
| 화학 및 물리 데이터 | |
| 공식 | C26H37N3O3 |
| 몰 질량 | 439.600 g·mol−1 |
| 3D 모델(JSmol) | |
| |
| |
| (표준) | |
MN-25(UR-12)는 Bristol-Myers [2]Squibb에 [1]의해 발명된 약물로, 말초 칸나비노이드 수용체의 합리적으로 선택적인 작용제 역할을 한다.K가i 11nM인 CB 수용체에는 중간2 친화력을 가지지만, K가i 245nM인 정신반응성1 CB 수용체에는 22배 낮은 친화력을 가진다.그러나 인돌 2-메틸 유도체는 CB에서1 K가i 8nM,[3][4] CB에서2 29nM으로 반전되어 CB에 대한2 선택성이 향상된 2-메틸 유도체의 일반적인 추세와 대조된다(cf. JWH-018 vs JWH-007, JWH-08).[5][6]
화학적으로는 또 다른 인돌-3-카르복사미드 합성 칸나비노이드인 Org 28611과 밀접하게 관련되어 있으나, JWH-200 또는 A-796,260과 같이 카르복사미드 상에서의 시클로알킬 치환과 시클로헥실메틸기가 모르포리닐에틸로 치환되어 있다.이러한 초기 화합물은 이후 많은 관련 인돌-3-카르복사미드 칸나비노이드 [7][8][9][10]배위자의 개발을 이끌었다.
「 」를 참조해 주세요.
레퍼런스
- ^ WO 어플리케이션 0158869, Leftheris K, Zhao R, Chen BC, Kiener P, Wu H, Pandit CR, Wrobleski S, Chen P, Hynes J, Longphre M, Noris DJ, Spergel S, Tokarski 수용체 Jinoid, Jnoid, Jnoid, Jn.브리스톨 마이어스 스퀴브에 서명하다
- ^ Zhao R, Wang B, Wu H, Hynes J, Leftheris K, Balasubramanian B, Barrish JC, Chen BC (20 December 2009). "Improved procedure for the preparation of 7-methoxy-2-methyl-1-(2-morpholinoethyl)-1H-indole-3-carboxylic acid, key intermediate in the synthesis of novel 3-amidoindole and indolopyridone cannabinoid ligands". Arkivoc. 2010 (6): 89–95. doi:10.3998/ark.5550190.0011.610.
- ^ Hynes J, Leftheris K, Wu H, Pandit C, Chen P, Norris DJ, et al. (September 2002). "C-3 Amido-indole cannabinoid receptor modulators". Bioorganic & Medicinal Chemistry Letters. 12 (17): 2399–402. doi:10.1016/S0960-894X(02)00466-3. PMID 12161142.
- ^ Wrobleski ST, Chen P, Hynes J, Lin S, Norris DJ, Pandit CR, Spergel S, Wu H, Tokarski JS, Chen X, Gillooly KM, Kiener PA, McIntyre KW, Patil-Koota V, Shuster DJ, Turk LA, Yang G, Leftheris K (May 2003). "Rational design and synthesis of an orally active indolopyridone as a novel conformationally constrained cannabinoid ligand possessing antiinflammatory properties". Journal of Medicinal Chemistry. 46 (11): 2110–6. doi:10.1021/jm020329q. PMID 12747783.
- ^ Huffman JW, Padgett LW (2005). "Recent developments in the medicinal chemistry of cannabimimetic indoles, pyrroles and indenes". Current Medicinal Chemistry. 12 (12): 1395–411. doi:10.2174/0929867054020864. PMID 15974991.
- ^ Manera C, Tuccinardi T, Martinelli A (April 2008). "Indoles and related compounds as cannabinoid ligands". Mini Reviews in Medicinal Chemistry. 8 (4): 370–87. doi:10.2174/138955708783955935. PMID 18473928.
- ^ Adam JM, Cairns J, Caulfield W, Cowley P, Cumming I, Easson M, et al. (2010). "Design, synthesis, and structure–activity relationships of indole-3-carboxamides as novel water soluble cannabinoid CB1 receptor agonists". MedChemComm. Royal Society of Chemistry. 1: 54–60. doi:10.1039/c0md00022a.
- ^ Kiyoi T, York M, Francis S, Edwards D, Walker G, Houghton AK, Cottney JE, Baker J, Adam JM (August 2010). "Design, synthesis, and structure-activity relationship study of conformationally constrained analogs of indole-3-carboxamides as novel CB1 cannabinoid receptor agonists". Bioorganic & Medicinal Chemistry Letters. 20 (16): 4918–21. doi:10.1016/j.bmcl.2010.06.067. PMID 20634067.
- ^ Moir EM, Yoshiizumi K, Cairns J, Cowley P, Ferguson M, Jeremiah F, Kiyoi T, Morphy R, Tierney J, Wishart G, York M, Baker J, Cottney JE, Houghton AK, McPhail P, Osprey A, Walker G, Adam JM (December 2010). "Design, synthesis, and structure-activity relationship study of bicyclic piperazine analogs of indole-3-carboxamides as novel cannabinoid CB1 receptor agonists". Bioorganic & Medicinal Chemistry Letters. 20 (24): 7327–30. doi:10.1016/j.bmcl.2010.10.061. PMID 21074434.
- ^ Blaazer AR, Lange JH, van der Neut MA, Mulder A, den Boon FS, Werkman TR, Kruse CG, Wadman WJ (October 2011). "Novel indole and azaindole (pyrrolopyridine) cannabinoid (CB) receptor agonists: design, synthesis, structure-activity relationships, physicochemical properties and biological activity". European Journal of Medicinal Chemistry. 46 (10): 5086–98. doi:10.1016/j.ejmech.2011.08.021. PMID 21885167.
추가 정보
- Hynes J, Leftheris K, Wu H, Pandit C, Chen P, Norris DJ, et al. (September 2002). "C-3 Amido-indole cannabinoid receptor modulators". Bioorganic & Medicinal Chemistry Letters. 12 (17): 2399–402. doi:10.1016/s0960-894x(02)00466-3. PMID 12161142.
- Frost JM, Dart MJ, Tietje KR, Garrison TR, Grayson GK, Daza AV, et al. (January 2010). "Indol-3-ylcycloalkyl ketones: effects of N1 substituted indole side chain variations on CB(2) cannabinoid receptor activity". Journal of Medicinal Chemistry. 53 (1): 295–315. doi:10.1021/jm901214q. PMID 19921781.
- Chin CL, Tovcimak AE, Hradil VP, Seifert TR, Hollingsworth PR, Chandran P, et al. (January 2008). "Differential effects of cannabinoid receptor agonists on regional brain activity using pharmacological MRI". British Journal of Pharmacology. 153 (2): 367–79. doi:10.1038/sj.bjp.0707506. PMC 2219521. PMID 17965748.


