호모 사피엔스 종의 단백질 코드 유전자
알파-액티닌-2 는 ACTN2 [5] 단백질 이다.이 유전자는 골격근과 심장근육에서 발현되는 알파-액틴 이소포름을 암호화하고 근섬유액틴을 얇은 필라멘트와 티틴 을 Z-디스크 에 고정하는 기능을 한다.
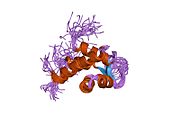
구조. 알파-액티닌-2는 아미노산 [6] [7] 각 분자는 막대 모양(길이 35 nm)이며 반평행 방식으로 균질화된다. 각 단량체는 2개의 칼포닌 호몰로지 도메인과 2개 의 C말단 EF 핸드 도메인과 4개 의 탠덤 스펙트린 형태의 반복으로 이루어진 [8] 인간 알파-액티닌 2의 3.5Ω에서의 고해상도 결정 구조가 최근에 [9] 알파 액티닌 은 스펙트린, 디스트로핀 , 우트로핀 및 [8] 핌브린 을 포함 한 액틴 결합 세포골격 단백질의 다양한 그룹을 나타내는 스펙트린 유전자 슈퍼패밀리에 속한다. 골격, 심장 및 평활근 아이소폼은 Z-디스크 와 유사한 밀도 있는 신체에 국소화되어 근섬유 액틴 필라멘트를 고정하는 데 도움이 됩니다. 알파-액티닌 2는 KCNA5,[10] [11] DLG1 ,[10] [12] MYOZ1 ,[13] GRIN2B ,[14] ADAM12 , ACTN3 ,[16] [17] PDLIM3 ,[18] [19] PKN ,[20] MYOT , TTN ,[21] [22] SYPO2 ,[23] L2 ,[24] [13] 상호작용 하는 것으로 나타났다 .
기능. 알파-액티닌-2의 주요 기능은 인지질에 [25] [26] Z-디스크 에서 인접한 육각류로부터 필라멘트 액틴 분자와 티틴 분자를 가교하는 것이다. Hampton 등의 연구에서 이러한 가교 작용은 60° 및 120° [27] 알파-액티닌-2는 또한 Z-디스크 에서 신호 전달 분자를 도킹하는 기능을 하며, 추가 연구도 알파-액티닌-2를 심장 이온 채널, 특히v [10] .5 의 결합에 포함시켰다.
임상적 의의 ACTN2 의 돌연변이는 확장형 심근증 [28] 심내막 섬유탄성증 뿐만 아니라 비후성 [29] 심근증 과 관련이 있다.알파-액티닌-2의 다양한 기능은 ACTN2 [30] 환자 의 다양한 임상 증상에 반영된다.
레퍼런스 ^ a b c GRCh38: 앙상블 릴리즈 89: ENSG000077522 - 앙상블 , 2017년 5월^ a b c GRCm38: 앙상블 릴리즈 89: ENSMUSG000052374 - 앙상블 , 2017년 5월^ "Human PubMed Reference:" . National Center for Biotechnology Information, U.S. National Library of Medicine .^ "Mouse PubMed Reference:" . National Center for Biotechnology Information, U.S. National Library of Medicine .^ "Entrez Gene: ACTN2 actinin, alpha 2" .^ "Protein Information – Basic Information: Protein COPaKB ID: P35609" . Cardiac Organellar Protein Atlas Knowledgebase . Archived from the original on 2015-04-13. Retrieved 2015-04-13 .^ Zong NC, Li H, Li H, Lam MP, Jimenez RC, Kim CS, Deng N, Kim AK, Choi JH, Zelaya I, Liem D, Meyer D, Odeberg J, Fang C, Lu HJ, Xu T, Weiss J, Duan H, Uhlen M, Yates JR, Apweiler R, Ge J, Hermjakob H, Ping P (October 2013). "Integration of cardiac proteome biology and medicine by a specialized knowledgebase" . Circulation Research . 113 (9): 1043–53. doi :10.1161/CIRCRESAHA.113.301151 . PMC 4076475 PMID 23965338 . ^ a b Luther PK (2009). "The vertebrate muscle Z-disc: sarcomere anchor for structure and signalling" . Journal of Muscle Research and Cell Motility . 30 (5–6): 171–85. doi :10.1007/s10974-009-9189-6 . PMC 2799012 PMID 19830582 . ^ Ribeiro Ede A, Pinotsis N, Ghisleni A, Salmazo A, Konarev PV, Kostan J, Sjöblom B, Schreiner C, Polyansky AA, Gkougkoulia EA, Holt MR, Aachmann FL, Zagrović B, Bordignon E, Pirker KF, Svergun DI, Gautel M, Djinović-Carugo K (December 2014). "The structure and regulation of human muscle α-actinin" . Cell . 159 (6): 1447–60. doi :10.1016/j.cell.2014.10.056 . PMC 4259493 PMID 25433700 . ^ a b c Eldstrom J, Choi WS, Steele DF, Fedida D (July 2003). "SAP97 increases Kv1.5 currents through an indirect N-terminal mechanism" . FEBS Letters . 547 (1–3): 205–11. doi :10.1016/S0014-5793(03)00668-9 PMID 12860415 . ^ Maruoka ND, Steele DF, Au BP, Dan P, Zhang X, Moore ED, Fedida D (May 2000). "alpha-actinin-2 couples to cardiac Kv1.5 channels, regulating current density and channel localization in HEK cells" . FEBS Letters . 473 (2): 188–94. doi :10.1016/S0014-5793(00)01521-0 PMID 10812072 . ^ Morris JA, Kandpal G, Ma L, Austin CP (July 2003). "DISC1 (Disrupted-In-Schizophrenia 1) is a centrosome-associated protein that interacts with MAP1A, MIPT3, ATF4/5 and NUDEL: regulation and loss of interaction with mutation" . Human Molecular Genetics . 12 (13): 1591–608. doi :10.1093/hmg/ddg162 PMID 12812986 . ^ a b Faulkner G, Pallavicini A, Comelli A, Salamon M, Bortoletto G, Ievolella C, Trevisan S, Kojic' S, Dalla Vecchia F, Laveder P, Valle G, Lanfranchi G (December 2000). "FATZ, a filamin-, actinin-, and telethonin-binding protein of the Z-disc of skeletal muscle" . The Journal of Biological Chemistry . 275 (52): 41234–42. doi :10.1074/jbc.M007493200 PMID 10984498 . ^ Wyszynski M, Lin J, Rao A, Nigh E, Beggs AH, Craig AM, Sheng M (January 1997). "Competitive binding of alpha-actinin and calmodulin to the NMDA receptor". Nature . 385 (6615): 439–42. doi :10.1038/385439a0 . PMID 9009191 . S2CID 4266742 . ^ Galliano MF, Huet C, Frygelius J, Polgren A, Wewer UM, Engvall E (May 2000). "Binding of ADAM12, a marker of skeletal muscle regeneration, to the muscle-specific actin-binding protein, alpha -actinin-2, is required for myoblast fusion" . The Journal of Biological Chemistry . 275 (18): 13933–9. doi :10.1074/jbc.275.18.13933 PMID 10788519 . ^ Chan Y, Tong HQ, Beggs AH, Kunkel LM (July 1998). "Human skeletal muscle-specific alpha-actinin-2 and -3 isoforms form homodimers and heterodimers in vitro and in vivo". Biochemical and Biophysical Research Communications . 248 (1): 134–9. doi :10.1006/bbrc.1998.8920 . PMID 9675099 . ^ Bang ML, Mudry RE, McElhinny AS, Trombitás K, Geach AJ, Yamasaki R, Sorimachi H, Granzier H, Gregorio CC, Labeit S (April 2001). "Myopalladin, a novel 145-kilodalton sarcomeric protein with multiple roles in Z-disc and I-band protein assemblies" . The Journal of Cell Biology . 153 (2): 413–27. doi :10.1083/jcb.153.2.413 . PMC 2169455 PMID 11309420 . ^ Pomiès P, Macalma T, Beckerle MC (October 1999). "Purification and characterization of an alpha-actinin-binding PDZ-LIM protein that is up-regulated during muscle differentiation" . The Journal of Biological Chemistry . 274 (41): 29242–50. doi :10.1074/jbc.274.41.29242 PMID 10506181 . ^ Mukai H, Toshimori M, Shibata H, Takanaga H, Kitagawa M, Miyahara M, Shimakawa M, Ono Y (February 1997). "Interaction of PKN with alpha-actinin" . The Journal of Biological Chemistry . 272 (8): 4740–6. doi :10.1074/jbc.272.8.4740 PMID 9030526 . ^ Salmikangas P, Mykkänen OM, Grönholm M, Heiska L, Kere J, Carpén O (July 1999). "Myotilin, a novel sarcomeric protein with two Ig-like domains, is encoded by a candidate gene for limb-girdle muscular dystrophy". Human Molecular Genetics . 8 (7): 1329–36. doi :10.1093/hmg/8.7.1329 . PMID 10369880 . ^ Young P, Ferguson C, Bañuelos S, Gautel M (March 1998). "Molecular structure of the sarcomeric Z-disk: two types of titin interactions lead to an asymmetrical sorting of alpha-actinin" . The EMBO Journal . 17 (6): 1614–24. doi :10.1093/emboj/17.6.1614 . PMC 1170509 PMID 9501083 . ^ Chunn CJ, Starr PR, Gilbert DN (August 1977). "Neutrophil toxicity of amphotericin B" . Antimicrobial Agents and Chemotherapy . 12 (2): 226–30. doi :10.1128/aac.12.2.226 . PMC 429889 PMID 900919 . ^ Linnemann A, van der Ven PF, Vakeel P, Albinus B, Simonis D, Bendas G, Schenk JA, Micheel B, Kley RA, Fürst DO (September 2010). "The sarcomeric Z-disc component myopodin is a multiadapter protein that interacts with filamin and alpha-actinin". European Journal of Cell Biology . 89 (9): 681–92. doi :10.1016/j.ejcb.2010.04.004 . PMID 20554076 . ^ Jani K, Schöck F (December 2007). "Zasp is required for the assembly of functional integrin adhesion sites" . The Journal of Cell Biology . 179 (7): 1583–97. doi :10.1083/jcb.200707045 . PMC 2373490 PMID 18166658 . ^ Young P, Gautel M (December 2000). "The interaction of titin and alpha-actinin is controlled by a phospholipid-regulated intramolecular pseudoligand mechanism" . The EMBO Journal . 19 (23): 6331–40. doi :10.1093/emboj/19.23.6331 . PMC 305858 PMID 11101506 . ^ Fukami K, Furuhashi K, Inagaki M, Endo T, Hatano S, Takenawa T (September 1992). "Requirement of phosphatidylinositol 4,5-bisphosphate for alpha-actinin function". Nature . 359 (6391): 150–2. doi :10.1038/359150a0 . PMID 1326084 . S2CID 4372960 . ^ Hampton CM, Taylor DW, Taylor KA (April 2007). "Novel structures for alpha-actinin:F-actin interactions and their implications for actin-membrane attachment and tension sensing in the cytoskeleton" . Journal of Molecular Biology . 368 (1): 92–104. doi :10.1016/j.jmb.2007.01.071 . PMC 1919418 PMID 17331538 . ^ Chiu C, Bagnall RD, Ingles J, Yeates L, Kennerson M, Donald JA, Jormakka M, Lind JM, Semsarian C (March 2010). "Mutations in alpha-actinin-2 cause hypertrophic cardiomyopathy: a genome-wide analysis" . Journal of the American College of Cardiology . 55 (11): 1127–35. doi :10.1016/j.jacc.2009.11.016 PMID 20022194 . ^ Mohapatra B, Jimenez S, Lin JH, Bowles KR, Coveler KJ, Marx JG, Chrisco MA, Murphy RT, Lurie PR, Schwartz RJ, Elliott PM, Vatta M, McKenna W, Towbin JA, Bowles NE (2003). "Mutations in the muscle LIM protein and alpha-actinin-2 genes in dilated cardiomyopathy and endocardial fibroelastosis". Molecular Genetics and Metabolism . 80 (1–2): 207–15. doi :10.1016/s1096-7192(03)00142-2 . PMID 14567970 . ^ Bagnall RD, Molloy LK, Kalman JM, Semsarian C (September 2014). "Exome sequencing identifies a mutation in the ACTN2 gene in a family with idiopathic ventricular fibrillation, left ventricular noncompaction, and sudden death" . BMC Medical Genetics . 15 (1): 99. doi :10.1186/s12881-014-0099-0 . PMC 4355500 PMID 25224718 .
추가 정보 Faulkner G, Lanfranchi G, Valle G (May 2001). "Telethonin and other new proteins of the Z-disc of skeletal muscle" . IUBMB Life . 51 (5): 275–82. doi :10.1080/152165401317190761 PMID 11699871 . Beggs AH, Byers TJ, Knoll JH, Boyce FM, Bruns GA, Kunkel LM (May 1992). "Cloning and characterization of two human skeletal muscle alpha-actinin genes located on chromosomes 1 and 11" . The Journal of Biological Chemistry . 267 (13): 9281–8. doi :10.1016/S0021-9258(19)50420-3 PMID 1339456 . Beggs AH, Phillips HA, Kozman H, Mulley JC, Wilton SD, Kunkel LM, Laing NG (August 1992). "A (CA)n repeat polymorphism for the human skeletal muscle alpha-actinin gene ACTN2 and its localization on the linkage map of chromosome 1". Genomics . 13 (4): 1314–5. doi :10.1016/0888-7543(92)90054-V . PMID 1505962 . Yürüker B, Niggli V (February 1992). "Alpha-actinin and vinculin in human neutrophils: reorganization during adhesion and relation to the actin network". Journal of Cell Science . 101 (2): 403–14. doi :10.1242/jcs.101.2.403 . PMID 1629252 . Pavalko FM, LaRoche SM (October 1993). "Activation of human neutrophils induces an interaction between the integrin beta 2-subunit (CD18) and the actin binding protein alpha-actinin". Journal of Immunology . 151 (7): 3795–807. PMID 8104223 . Yoshida M, Westlin WF, Wang N, Ingber DE, Rosenzweig A, Resnick N, Gimbrone MA (April 1996). "Leukocyte adhesion to vascular endothelium induces E-selectin linkage to the actin cytoskeleton" . The Journal of Cell Biology . 133 (2): 445–55. doi :10.1083/jcb.133.2.445 . PMC 2120789 PMID 8609175 . Wyszynski M, Lin J, Rao A, Nigh E, Beggs AH, Craig AM, Sheng M (January 1997). "Competitive binding of alpha-actinin and calmodulin to the NMDA receptor". Nature . 385 (6615): 439–42. doi :10.1038/385439a0 . PMID 9009191 . S2CID 4266742 . Mukai H, Toshimori M, Shibata H, Takanaga H, Kitagawa M, Miyahara M, Shimakawa M, Ono Y (February 1997). "Interaction of PKN with alpha-actinin" . The Journal of Biological Chemistry . 272 (8): 4740–6. doi :10.1074/jbc.272.8.4740 PMID 9030526 . Young P, Ferguson C, Bañuelos S, Gautel M (March 1998). "Molecular structure of the sarcomeric Z-disk: two types of titin interactions lead to an asymmetrical sorting of alpha-actinin" . The EMBO Journal . 17 (6): 1614–24. doi :10.1093/emboj/17.6.1614 . PMC 1170509 PMID 9501083 . Zhang S, Ehlers MD, Bernhardt JP, Su CT, Huganir RL (August 1998). "Calmodulin mediates calcium-dependent inactivation of N-methyl-D-aspartate receptors" . Neuron . 21 (2): 443–53. doi :10.1016/S0896-6273(00)80553-X PMID 9728925 . S2CID 18234477 . Krupp JJ, Vissel B, Thomas CG, Heinemann SF, Westbrook GL (February 1999). "Interactions of calmodulin and alpha-actinin with the NR1 subunit modulate Ca2+-dependent inactivation of NMDA receptors" . The Journal of Neuroscience . 19 (4): 1165–78. doi :10.1523/JNEUROSCI.19-04-01165.1999 PMC 6786025 PMID 9952395 . Zhou Q, Ruiz-Lozano P, Martone ME, Chen J (July 1999). "Cypher, a striated muscle-restricted PDZ and LIM domain-containing protein, binds to alpha-actinin-2 and protein kinase C" . The Journal of Biological Chemistry . 274 (28): 19807–13. doi :10.1074/jbc.274.28.19807 PMID 10391924 . Faulkner G, Pallavicini A, Formentin E, Comelli A, Ievolella C, Trevisan S, Bortoletto G, Scannapieco P, Salamon M, Mouly V, Valle G, Lanfranchi G (July 1999). "ZASP: a new Z-band alternatively spliced PDZ-motif protein" . The Journal of Cell Biology . 146 (2): 465–75. doi :10.1083/jcb.146.2.465 . PMC 3206570 PMID 10427098 . Tiso N, Majetti M, Stanchi F, Rampazzo A, Zimbello R, Nava A, Danieli GA (November 1999). "Fine mapping and genomic structure of ACTN2, the human gene coding for the sarcomeric isoform of alpha-actinin-2, expressed in skeletal and cardiac muscle". Biochemical and Biophysical Research Communications . 265 (1): 256–9. doi :10.1006/bbrc.1999.1661 . PMID 10548523 . Nikolopoulos SN, Spengler BA, Kisselbach K, Evans AE, Biedler JL, Ross RA (January 2000). "The human non-muscle alpha-actinin protein encoded by the ACTN4 gene suppresses tumorigenicity of human neuroblastoma cells" . Oncogene . 19 (3): 380–6. doi :10.1038/sj.onc.1203310 PMID 10656685 . Galliano MF, Huet C, Frygelius J, Polgren A, Wewer UM, Engvall E (May 2000). "Binding of ADAM12, a marker of skeletal muscle regeneration, to the muscle-specific actin-binding protein, alpha -actinin-2, is required for myoblast fusion" . The Journal of Biological Chemistry . 275 (18): 13933–9. doi :10.1074/jbc.275.18.13933 PMID 10788519 . Maruoka ND, Steele DF, Au BP, Dan P, Zhang X, Moore ED, Fedida D (May 2000). "alpha-actinin-2 couples to cardiac Kv1.5 channels, regulating current density and channel localization in HEK cells" . FEBS Letters . 473 (2): 188–94. doi :10.1016/S0014-5793(00)01521-0 PMID 10812072 . Kotaka M, Kostin S, Ngai S, Chan K, Lau Y, Lee SM, Li H, Ng EK, Schaper J, Tsui SK, Fung K, Lee C, Waye MM (June 2000). "Interaction of hCLIM1, an enigma family protein, with alpha-actinin 2". Journal of Cellular Biochemistry . 78 (4): 558–65. doi :10.1002/1097-4644(20000915)78:4<558::AID-JCB5>3.0.CO;2-I . PMID 10861853 . S2CID 84122657 . Parast MM, Otey CA (August 2000). "Characterization of palladin, a novel protein localized to stress fibers and cell adhesions" . The Journal of Cell Biology . 150 (3): 643–56. doi :10.1083/jcb.150.3.643 . PMC 2175193 PMID 10931874 . Faulkner G, Pallavicini A, Comelli A, Salamon M, Bortoletto G, Ievolella C, Trevisan S, Kojic' S, Dalla Vecchia F, Laveder P, Valle G, Lanfranchi G (December 2000). "FATZ, a filamin-, actinin-, and telethonin-binding protein of the Z-disc of skeletal muscle" . The Journal of Biological Chemistry . 275 (52): 41234–42. doi :10.1074/jbc.M007493200 PMID 10984498 . 외부 링크
PDB 갤러리
1h8b : 알파-액티닌에서 EF-HANDS 3,4 / 티틴에서 Z-반복 7
1hci : α-액티닌의 로드 도메인의 결정 구조
1quu : 알파액티닌으로부터의 2개의 중심 스펙트린상 반복체의 결정 구조
1tjt : 인간 알파-액틴 이소폼 3의 X선 구조(2)2A 해상도
1wku : 인간 알파-액티닌 이소폼 3의 고해상도 구조