에모딘
Emodin
 | |
 | |
| 이름 | |
|---|---|
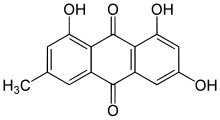
| 우선 IUPAC 이름 1,3,8-트리히드록시-6-메틸안트라센-9,10-디온 | |
| 기타 이름 6-메틸-1,3,8-트리히드록시안트라퀴논 | |
| 식별자 | |
3D 모델(JSmol) | |
| 체비 | |
| 첸블 | |
| 켐스파이더 | |
| 드러그뱅크 | |
| ECHA 정보 카드 | 100.007.509 |
| 케그 | |
PubChem CID | |
| 유니 | |
CompTox 대시보드 (EPA ) | |
| |
| |
| 특성. | |
| C15H10O5 | |
| 몰 질량 | 270.240g/140g−1/140g/140g |
| 외모 | 오렌지 솔리드 |
| 밀도 | 1.583±0.06g/cm3 |
| 녹는점 | 256~257°C(493~495°F, 529~530K) |
달리 명시되지 않은 한 표준 상태(25°C[77°F], 100kPa)의 재료에 대한 데이터가 제공됩니다. | |
에모딘(6-메틸-1,3,8-트리히드록시안트라퀴논)은 안트라퀴논과의 화합물로 대황, 버크손 및 일본 매듭풀(레이노트리아 자포니카)에서 분리할 수 있다.Polygonum cuspidatum).[1]에모딘은 특히 대황(Rheum palmatum), 매듭풀, 매듭풀(Polygonum cuspidatum 및 multilorum) 뿌리뿐만 아니라 하와이 'au'auki cassia' 씨앗 또는 커피 잡초(Semen cassia)[2]에 풍부합니다.이것은 류마티스 팔마텀 [3]L에서 특별히 분리된다.그것은 또한 아스페르길루스속, 피레노차에타속, 페스타로티옵시스속을 포함한 많은 종류의 곰팡이에 의해 생산된다.일반적인 이름은 류마티스 오스트랄의 분류학적 동의어인 류마티스 에모디(Himalayan Rhumb)에서 유래했으며 동의어는 에모돌, 프랑굴라 에모딘, 류마티스 에모딘, 3-메틸-1, 6,8-트리히드록시안트라퀴논, 슈트겔브, 페르시아 베리 [4]호수를 포함한다.
약리학
에모딘은 류마티스 팔마툼, 폴리곤 큐스피다툼, 폴리곤 다염소 등 전통 한의학(TCM)에 사용되는 여러 식물의 활성 성분입니다.하제, 항균,[5][6] 항염증 효과 등 다양한 작용을 하며, 항바이러스 [9][10]TCM 제제의 주요 활성 성분 중 하나인 SARS-CoV-2와 같은 코로나 바이러스에 대한 잠재적 항바이러스 활성을 가진 것으로 확인되었다.[7][8]
Spike-ACE2 단백질 [2]복합체 형성에 대한 억제 메커니즘을 조사하기 위해 계산 연구가 수행되었다.구체적으로는 SARS-Cov-2에 [2]대한 추가 조사를 자극한 증거가 있는 SARS-Cov-1에 의한 감염 방지에 용량 의존적인 억제가 보인다.
에모딘은 감염된 [11]세포에서 바이러스를 방출하는 데 결정적인 역할을 할 수 있는 단백질 3a의 이온 채널을 억제하는 것으로 나타났다.
식물 목록
다음 식물종이 에모딘을 생산한다.
- 아카리파오스트랄리스[12]
- 카시아오시덴탈리스[13]
- 카시아시아메아메아[14]
- 쯔쯔카미[15]
- 글로소스테몬 브루기에리[16]
- 카리메리스 인디카[17]
- 폴리곤룸하이폴루쿰[18]
- 레이노트리아 자포니카.팔로피아 자포니카([19]syn.Polygonum cuspidatum[20])
- 알더리프버크손[21] 람누스 알니폴리아
- 럼누스 카타르티카, 통큰큰가시[21]
- 류마티스팔마툼[22]
- 루멕스네팔렌시스[23]
- Senna obtusifolia[24] (싱크).카시아오투시폴리아[25])
- 티엘라비아아테르모필라[26]
- 벤틸라고 마드라스파타나[27]
보충 상태
레퍼런스
- ^ 도란드의학사전(1938년)
- ^ a b c Dellafiora L, Dorne JL, Galaverna G, Dall'Asta C (2020). "Preventing the Interaction between Coronaviruses Spike Protein and Angiotensin I Converting Enzyme 2: An in Silico Mechanistic Case Study on Emodin as a Potential Model Compound". Applied Sciences. 10 (18): 6358. doi:10.3390/app10186358. S2CID 224994102.
- ^ Tsay HS, Shyur LF, Agrawal DC, Wu YC, Wang SY (3 November 2017). Medicinal Plants – Recent Advances in Research and Development. Singapore: Springer Singapore. p. 339. ISBN 978-981-10-5978-0.
- ^ PubChem의 CID 3220
- ^ Dong X, Fu J, Yin X, Cao S, Li X, Lin L, Ni J (August 2016). "Emodin: A Review of its Pharmacology, Toxicity and Pharmacokinetics". Phytotherapy Research. 30 (8): 1207–18. doi:10.1002/ptr.5631. PMC 7168079. PMID 27188216.
- ^ Monisha BA, Kumar N, Tiku AB (2016). "Emodin and Its Role in Chronic Diseases". Advances in Experimental Medicine and Biology. 928: 47–73. doi:10.1007/978-3-319-41334-1_3. ISBN 978-3-319-41332-7. PMID 27671812.
- ^ Ho TY, Wu SL, Chen JC, Li CC, Hsiang CY (May 2007). "Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction". Antiviral Research. 74 (2): 92–101. doi:10.1016/j.antiviral.2006.04.014. PMC 7114332. PMID 16730806.
- ^ Zhou Y, Hou Y, Shen J, Huang Y, Martin W, Cheng F (2020). "Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2". Cell Discovery. 6: 14. doi:10.1038/s41421-020-0153-3. PMC 7073332. PMID 32194980.
- ^ Wang CH, Zhong Y, Zhang Y, Liu JP, Wang YF, Jia WN, et al. (February 2016). "A network analysis of the Chinese medicine Lianhua-Qingwen formula to identify its main effective components". Molecular BioSystems. 12 (2): 606–13. doi:10.1039/c5mb00448a. PMID 26687282.
- ^ Runfeng L, Yunlong H, Jicheng H, Weiqi P, Qinhai M, Yongxia S, et al. (June 2020). "Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2)". Pharmacological Research. 156: 104761. doi:10.1016/j.phrs.2020.104761. PMC 7102548. PMID 32205232.
- ^ Schwarz S, Wang K, Yu W, Sun B, Schwarz W (April 2011). "Emodin inhibits current through SARS-associated coronavirus 3a protein". Antiviral Research. 90 (1): 64–9. doi:10.1016/j.antiviral.2011.02.008. PMC 7114100. PMID 21356245.
- ^ Wang XL, Yu KB, Peng SL (June 2008). "[Chemical constituents of aerial part of Acalypha australis]" [Chemical Constituents of Aerial Part of Acalypha australis]. Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China Journal of Chinese Materia Medica (in Chinese). 33 (12): 1415–7. PMID 18837345.
- ^ Yadav JP, Arya V, Yadav S, Panghal M, Kumar S, Dhankhar S (June 2010). "Cassia occidentalis L.: a review on its ethnobotany, phytochemical and pharmacological profile". Fitoterapia. 81 (4): 223–30. doi:10.1016/j.fitote.2009.09.008. PMID 19796670.
- ^ Nsonde Ntandou GF, Banzouzi JT, Mbatchi B, Elion-Itou RD, Etou-Ossibi AW, Ramos S, et al. (January 2010). "Analgesic and anti-inflammatory effects of Cassia siamea Lam. stem bark extracts". Journal of Ethnopharmacology. 127 (1): 108–11. doi:10.1016/j.jep.2009.09.040. PMID 19799981.
- ^ Faculty of Pharmacy and Biochemistry, University of Zagreb, A. Kovačića 1, 10000 Zagreb, Croatia b Dipartimento di Scienze del Farmaco, Università degli Studi "G. d’Annunzio" di Chieti-Pescara, Via dei Vestini 31, 66100 Chieti, Italy (April 2012). "Anthraquinone profiles, antioxidant and antimicrobial properties of Frangula rupestris (Scop.) Schur and Frangula alnus Mill. bark". Food Chemistry. 131 (4): 1174–1180. doi:10.1016/j.foodchem.2011.09.094.
{{cite journal}}: CS1 maint: 여러 이름: 작성자 목록(링크) - ^ Meselhy MR (August 2003). "Constituents from Moghat, the Roots of Glossostemon bruguieri (Desf.)". Molecules. 8 (8): 614–621. doi:10.3390/80800614. PMC 6146927.
- ^ Wang G, Wang GK, Liu JS, Yu B, Wang F, Liu JK (April 2010). "[Studies on the chemical constituents of Kalimeris indica]" [Studies on the Chemical Constituents of Kalimeris indica]. Zhong Yao Cai = Zhongyaocai = Journal of Chinese Medicinal Materials (in Chinese). 33 (4): 551–4. PMID 20845783.
- ^ Chao PM, Kuo YH, Lin YS, Chen CH, Chen SW, Kuo YH (April 2010). "The metabolic benefits of Polygonum hypoleucum Ohwi in HepG2 cells and Wistar rats under lipogenic stress". Journal of Agricultural and Food Chemistry. 58 (8): 5174–80. doi:10.1021/jf100046h. PMID 20230058.
- ^ "Reynoutria japonica (Polygonaceae)". Dr. Duke's Phytochemical and Ethnobotanical Databases. U.S. Department of Agriculture.
- ^ Ban SH, Kwon YR, Pandit S, Lee YS, Yi HK, Jeon JG (January 2010). "Effects of a bio-assay guided fraction from Polygonum cuspidatum root on the viability, acid production and glucosyltranferase of mutans streptococci". Fitoterapia. 81 (1): 30–4. doi:10.1016/j.fitote.2009.06.019. PMID 19616082.
- ^ a b Sacerdote, Allison B.; King, Richard B. (2014). "Direct Effects of an Invasive European Buckthorn Metabolite on Embryo Survival and Development in Xenopus laevis and Pseudacris triseriata" (PDF). Journal of Herpetology. 48 (1): 51–58. doi:10.1670/12-066. S2CID 62818226.
- ^ Liu A, Chen H, Wei W, Ye S, Liao W, Gong J, et al. (July 2011). "Antiproliferative and antimetastatic effects of emodin on human pancreatic cancer". Oncology Reports. 26 (1): 81–9. doi:10.3892/or.2011.1257. PMID 21491088.
- ^ Gautam R, Karkhile KV, Bhutani KK, Jachak SM (October 2010). "Anti-inflammatory, cyclooxygenase (COX)-2, COX-1 inhibitory, and free radical scavenging effects of Rumex nepalensis". Planta Medica. 76 (14): 1564–9. doi:10.1055/s-0030-1249779. PMID 20379952.
- ^ "Senna obtusifolia (Fabaceae)". Dr. Duke's Phytochemical and Ethnobotanical Databases. U.S. Department of Agriculture.
- ^ Yang YC, Lim MY, Lee HS (December 2003). "Emodin isolated from Cassia obtusifolia (Leguminosae) seed shows larvicidal activity against three mosquito species". Journal of Agricultural and Food Chemistry. 51 (26): 7629–31. doi:10.1021/jf034727t. PMID 14664519.
- ^ Kusari S, Zühlke S, Kosuth J, Cellárová E, Spiteller M (October 2009). "Light-independent metabolomics of endophytic Thielavia subthermophila provides insight into microbial hypericin biosynthesis". Journal of Natural Products. 72 (10): 1825–35. doi:10.1021/np9002977. PMID 19746917.
- ^ Ghosh S, Das Sarma M, Patra A, Hazra B (September 2010). "Anti-inflammatory and anticancer compounds isolated from Ventilago madraspatana Gaertn., Rubia cordifolia Linn. and Lantana camara Linn". The Journal of Pharmacy and Pharmacology. 62 (9): 1158–66. doi:10.1111/j.2042-7158.2010.01151.x. PMID 20796195. S2CID 25769269.
- ^ The British Pharmacopoeia Secretariat (2009). "Index, BP 2009" (PDF). Archived from the original (PDF) on 11 April 2009. Retrieved 20 April 2010.


