독산트린
Doxanthrine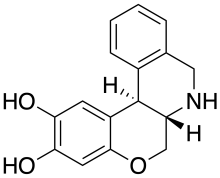 | |
| 임상자료 | |
|---|---|
| ATC 코드 |
|
| 식별자 | |
| |
| 펍켐 CID | |
| 켐스파이더 | |
| 화학 및 물리적 데이터 | |
| 공식 | C16H15NO3 |
| 어금질량 | 269.300 g·190−1 |
| 3D 모델(JSmol) | |
| |
| |
| (iii) | |
독산트린은 도파민 D1 수용체의 강력하고 선택적인 완전 작용제인 합성 화합물이다.[1][2]독산트린은 파킨슨병의 6-히드록시도파민 랫드 모델에서 횡방향 회전 생산에 구술적으로 적극적인 것으로 나타났다.[3]
참조
- ^ Cueva JP, Giorgioni G, Grubbs RA, Chemel BR, Watts VJ, Nichols DE (November 2006). "trans-2,3-dihydroxy-6a,7,8,12b-tetrahydro-6H-chromeno[3,4-c]isoquinoline: synthesis, resolution, and preliminary pharmacological characterization of a new dopamine D1 receptor full agonist". Journal of Medicinal Chemistry. 49 (23): 6848–57. doi:10.1021/jm0604979. PMID 17154515.
- ^ Przybyla JA, Cueva JP, Chemel BR, Hsu KJ, Riese DJ, McCorvy JD, Chester JA, Nichols DE, Watts VJ (February 2009). "Comparison of the enantiomers of (±)-doxanthrine, a high efficacy full dopamine D1 receptor agonist, and a reversal of enantioselectivity at D1 versus alpha2C adrenergic receptors". European Neuropsychopharmacology. 19 (2): 138–46. doi:10.1016/j.euroneuro.2008.10.002. PMC 2636714. PMID 19028082.
- ^ McCorvey JD, Watts VJ, Nichols DE (July 2012). "Comparison of the D1 dopamine full agonists, dihydrexidine and doxanthrine, in the 6-OHDA rat model of Parkinson's disease". Psychopharmacology. 222 (1): 81–87. doi:10.1007/s00213-011-2625-5. PMID 22222862. S2CID 7641172.


