이소발레릴-CoA
Isovaleryl-CoA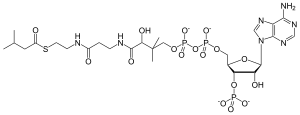 | |
| 이름 | |
|---|---|
| 선호 IUPAC 이름 O1-{[(2R,3S,4R,5R)-5-(6-Amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methyl} O3-[(3R)-3-hydroxy-4-{[3-({2-[(3-methylbutanoyl)sulfanyl]ethyl}amino)-3-oxopropyl]amino}-2-methyl-4-oxobutyl] dihydrogen diphosphate | |
| 식별자 | |
3D 모델(JSmol) | |
| 체비 | |
| 켐스파이더 | |
| 메슈 | 이소발레릴-코엔자임+a |
펍켐 CID | |
CompTox 대시보드 (EPA) | |
| |
| |
| 특성. | |
| C26H44N7O17P3S | |
| 어금질량 | 851.652 g/190 |
달리 명시된 경우를 제외하고, 표준 상태(25°C [77°F], 100 kPa)의 재료에 대한 데이터가 제공된다. | |
| Infobox 참조 자료 | |
Isovaleryl-CoA라고도 알려진 Isovaleryl-coenzyme A는 분기 체인 아미노산의 신진대사의 중간이다.[1][2][3]null
루신 대사
참고 항목
참조
- ^ a b c Wilson JM, Fitschen PJ, Campbell B, Wilson GJ, Zanchi N, Taylor L, Wilborn C, Kalman DS, Stout JR, Hoffman JR, Ziegenfuss TN, Lopez HL, Kreider RB, Smith-Ryan AE, Antonio J (February 2013). "International Society of Sports Nutrition Position Stand: beta-hydroxy-beta-methylbutyrate (HMB)". Journal of the International Society of Sports Nutrition. 10 (1): 6. doi:10.1186/1550-2783-10-6. PMC 3568064. PMID 23374455.
- ^ a b c Kohlmeier M (May 2015). "Leucine". Nutrient Metabolism: Structures, Functions, and Genes (2nd ed.). Academic Press. pp. 385–388. ISBN 978-0-12-387784-0. Retrieved 6 June 2016.
Energy fuel: Eventually, most Leu is broken down, providing about 6.0kcal/g. About 60% of ingested Leu is oxidized within a few hours ... Ketogenesis: A significant proportion (40% of an ingested dose) is converted into acetyl-CoA and thereby contributes to the synthesis of ketones, steroids, fatty acids, and other compounds
그림 8.57: L-루신의 대사