게르마크렌
Germacrene | |
| 이름 | |
|---|---|
| IUPAC 이름 (1E,5E,8S)-1,5-디메틸-8-(prop-1-en-2-yl)사이클로데카-1,5-diene | |
기타 이름
| |
| 식별자 | |
| |
3D 모델(JSmol) | |
| 6500908 (A) 1864177 (D) | |
| 체비 |
|
| 켐벨 |
|
| 켐스파이더 | |
| 케그 | |
펍켐 CID | |
| |
| |
| 특성. | |
| C15H24 | |
| 어금질량 | 204.35 g/190 |
| 밀도 | 0.793 g/mL |
| 비등점 | 236.4°C(457.5°F, 509.5K) |
달리 명시된 경우를 제외하고, 표준 상태(25°C [77°F], 100 kPa)의 재료에 대한 데이터가 제공된다. | |
| Infobox 참조 자료 | |
 | |
| 이름 | |
|---|---|
| IUPAC 이름 (S,1Z,6Z)-8-이소프로필-1-메틸-5-메틸리덴사이클로데카-1,6-다이엔 | |
| 기타 이름 1-메틸-5-메틸렌-8-(1-메틸레틸)-1,6-사이클로데카디엔 | |
| 식별자 | |
| |
3D 모델(JSmol) | |
| 켐스파이더 | |
펍켐 CID | |
| |
| |
| 특성. | |
| C15H24 | |
| 어금질량 | 204.35 g/190 |
달리 명시된 경우를 제외하고, 표준 상태(25°C [77°F], 100 kPa)의 재료에 대한 데이터가 제공된다. | |
| Infobox 참조 자료 | |
Germacrenes는 휘발성 유기 탄화수소의 한 종류, 특히 세스키테르펜이다. 제르마크레네는 곤충 페로몬의 역할도 하지만 항균성과 살충성을 위해 많은 식물 종에서 생산된다. 두 개의 두드러진 분자는 제르마크렌 A와 제르마크렌 D이다.
구조물들
게르마크렌은 5개의 이소머를 가지고 있다.
 |  |  | 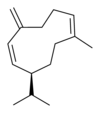 | 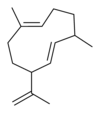 |
| 게르마크렌 A | 게르마크렌 B | 게르마크렌 C | 게르마크렌 D | 게르마크렌 E |
자연발생
붉은 데드네틀(Lamium purpleum)[1]과 헤지네틀(Genus Stachys)[2]의 에센셜 오일은 클라우세나 아니사타처럼 게르마크렌 D의 함량이 높은 것이 특징이다.
참조
- ^ Flamini, G.; Cioni, P. L.; Morelli, I. (2005). "Composition of the essential oils and in vivo emission of volatiles of four Lamium species from Italy: L. purpureum, L. hybridum, L. bifidum and L. amplexicaule". Food Chemistry. 91 (1): 63–68. doi:10.1016/j.foodchem.2004.05.047.
- ^ Morteza‐Semnani, K.; Akbarzadeh, M.; Changizi, Sh. (2005-11-01). "Essential oils composition of Stachys byzantina, S. inflata, S. lavandulifolia and S. laxa from Iran". Flavour and Fragrance Journal. 21 (2): 300–303. doi:10.1002/ffj.1594.
추가 읽기
일반
- Adio, A. M. (2009). "Germacrenes A–E and related compounds: thermal, photochemical and acid induced transannular cyclizations". Tetrahedron. 65 (8): 1533–1552. doi:10.1016/j.tet.2008.11.050.
게르마크렌 A
- Deguerry, F.; Pastore, L.; Wu, S.; Clark, A.; Chappell, J.; Schalk, M. (2006-10-15). "The diverse sesquiterpene profile of patchouli, Pogostemon cablin, is correlated with a limited number of sesquiterpene synthases". Archives of Biochemistry and Biophysics. 454 (2): 123–136.
- Omura, H.; Honda, K.; Feeny, P. (2006-09-01). "From terpenoids to aliphatic acids: further evidence for late-instar switch in osmeterial defense as a characteristic trait of swallowtail butterflies in the tribe Papilionini". Journal of Chemical Ecology. 32 (9): 1999–2012.
- Forcat, S.; Allemann, R. K. (2006-07-07). "Stabilisation of transition states prior to and following eudesmane cation in aristolochene synthase". Organic and Biomolecular Chemistry. 4 (13): 2563–2567.
- Bertea, C. M.; Voster, A.; Verstappen, F. W.; Maffei, M.; Beekwilder, J.; Bouwmeester, H. J. (2006-04-15). "Isoprenoid biosynthesis in Artemisia annua: cloning and heterologous expression of a germacrene A synthase from a glandular trichome cDNA library". Archives of Biochemistry and Biophysics. 448 (1–2): 3–12.
- Lou, Y; Baldwin, I. T. (2006-03-01). "Silencing of a germin-like gene in Nicotiana attenuata improves performance of native herbivores". Plant Physiology. 140 (3): 1126–1136.
- Chang, Y.-J.; Jin, J.; Nam, H.-Y.; Kim, S.-U. (2005-03-01). "Point mutation of (+)-germacrene A synthase from Ixeris dentata". Biotechnology Letters. 27 (5): 285–288.
게르마크렌 D
- Rivero Cruz, B.; Rivero Cruz, I; Rodríguez, J. M.; Cerda García-Rojas, C. M.; Mata, R. (2006-08-01). "Qualitative and quantitative analysis of the active components of the essential oil from 'Brickellia veronicaefolia by nuclear magnetic resonance spectroscopy". Journal of Natural Products. 69 (8): 1172–1176.
- Yang, F.-Q.; Li, S.-P.; Chen, Y.; Lao, S.-C.; Wang, Y.-T.; Dong, T.-T.; Tsim, K.-W. (2005-09-15). "Identification and quantitation of eleven sesquiterpenes in three species of Curcuma rhizomes by pressurized liquid extraction and gas chromatography-mass spectrometry". Journal of Pharmaceutical and Biomedical Analysis. 39 (3–4): 552–558.
- Umlauf, D.; Zapp, J.; Becker, H.; Adam, K. P. (2004-09-01). "Biosynthesis of the irregular monoterpene artemisia ketone, the sesquiterpene germacrene D and other isoprenoids in Tanacetum vulgare L. (Asteraceae)". Phytochemistry. 65 (17): 2463–2470.
- Agnihotri, V. K.; Thappa, R. K.; Meena, B.; Kapahi, B. K.; Saxena, R. K.; Qazi, G. N.; Agarwal, S. G. (2004-08-01). "Essential oil composition of aerial parts of Angelica glauca growing wild in North-West Himalaya (India)". Phytochemistry. 65 (16): 2411–2413.
- Raal, A.; Paaver, U.; Arak, E.; Orav, A. (2004). "Content and composition of the essential oil of Thymus serpyllum L. growing wild in Estonia". Medicina (Kaunas). 40 (8): 795–800.
- He, X.; Cane, D. E. (2004-03-10). "Mechanism and stereochemistry of the germacradienol/germacrene D synthase of Streptomyces coelicolor A3(2)". Journal of the American Chemical Society. 126 (9): 2678–2679.
- Arimura, G.-I.; Huber, D. P. W.; Bohlmann, J. (2004). "Forest tent caterpillars (Malacosoma disstria) induce local and systemic diurnal emissions of terpenoid volatiles in hybrid poplar (Populus trichocarpa × deltoides): cDNA cloning, functional characterization, and patterns of gene expression of (−)-germacrene D synthase, PtdTPS1". The Plant Journal. 37: 603–616.


