노르시클로아르테놀
Norcycloartenol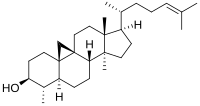 | |
| 이름 | |
|---|---|
| 기타 이름 4α,14α-γ-9β,19-γ-5α-콜레스트-24-en-3β-ol | |
| 식별자 | |
3D 모델(JSmol) | |
| 켐스파이더 | |
펍켐 CID | |
| |
| |
| 특성. | |
| C29H48O | |
| 몰 질량 | 412.702 g·mol−1 |
달리 명시된 경우를 제외하고 표준 상태(25 °C [77 °F], 100 kPa)의 재료에 대한 데이터가 제공됩니다. | |
29-노르시클로아르테놀[note 1]() 또는 4α,14α-디메틸-9β,[1][2][3][4]19-시클로 5α,14α-디메틸-9β,19-시클로-5α-콜레스트-24-en-3β-ol.대사 경로에서 사이클로아르테놀의 탈메틸화로부터 전환된 다음, 9,19-시클로프로필-고리 개방 반응이 29-노를로스테롤로 [5]발생합니다.
메모
- ^ 동물학과 식물학은 스테로이드 사이드 체인의 번호가 다릅니다, 24에서1 28, 24에서2 29.
레퍼런스
- ^ Zheng, S; Ma, Z; Ye, J; Wang, G; Wang, R; Zhou, H; Zeng, S; Jiang, H (1 January 2014). "Determination of three triterpene alcohols in rat plasma after oral administration of pollen of Brassica campestris based on the utilization of fetal bovine serum as surrogate matrix". Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences. 944: 11–7. doi:10.1016/j.jchromb.2013.10.044. PMID 24291607.
- ^ Bellamine, A; Mangla, AT; Dennis, AL; Nes, WD; Waterman, MR (January 2001). "Structural requirements for substrate recognition of Mycobacterium tuberculosis 14 alpha-demethylase: implications for sterol biosynthesis". Journal of Lipid Research. 42 (1): 128–36. doi:10.1016/S0022-2275(20)32344-0. PMID 11160374.
- ^ Bayer, M; Proksch, P; Felsner, I; Brenden, H; Kohne, Z; Walli, R; Duong, TN; Götz, C; Krutmann, J; Grether-Beck, S (November 2011). "Photoprotection against UVAR: effective triterpenoids require a lipid raft stabilizing chemical structure". Experimental Dermatology. 20 (11): 955–8. doi:10.1111/j.1600-0625.2011.01350.x. PMID 21824200. S2CID 28583844.
- ^ Hartmann, MA; Perret, AM; Carde, JP; Cassagne, C; Moreau, P (8 August 2002). "Inhibition of the sterol pathway in leek seedlings impairs phosphatidylserine and glucosylceramide synthesis but triggers an accumulation of triacylglycerols". Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 1583 (3): 285–96. doi:10.1016/s1388-1981(02)00249-4. PMID 12176396.
- ^ "Rosa chinensis plant sterol biosynthesis II". pmn.plantcyc.org.


