Indium gallium arsenide
Indium gallium arsenide (InGaAs) (alternatively gallium indium arsenide, GaInAs) is a ternary alloy (chemical compound) of indium arsenide (InAs) and gallium arsenide (GaAs). Indium and gallium are (group III) elements of the periodic table while arsenic is a (group V) element. Alloys made of these chemical groups are referred to as "III-V" compounds. InGaAs has properties intermediate between those of GaAs and InAs. InGaAs is a room-temperature semiconductor with applications in electronics and photonics.
The principal importance of GaInAs is its application as a high-speed, high sensitivity photodetector of choice for optical fiber telecommunications.[1]
Nomenclature
Indium gallium arsenide (InGaAs) and gallium-indium arsenide (GaInAs) are used interchangeably. According to IUPAC standards[2] the preferred nomenclature for the alloy is GaxIn1-xAs where the group-III elements appear in order of increasing atomic number, as in the related alloy system AlxGa1-xAs. By far, the most important alloy composition from technological and commercial standpoints is Ga0.47In0.53As, which can be deposited in single crystal form on indium phosphide (InP).
Materials synthesis
GaInAs is not a naturally-occurring material. Single crystal material is required for electronic and photonic device applications. Pearsall and co-workers were the first to describe single-crystal epitaxial growth of In0.53Ga0.47As on (111)-oriented [3] and on (100)-oriented [4] InP substrates. Single crystal material in thin-film form can be grown by epitaxy from the liquid-phase (LPE), vapour-phase (VPE), by molecular beam epitaxy (MBE), and by metalorganic chemical vapour deposition (MO-CVD).[5] Today, most commercial devices are produced by MO-CVD or by MBE.
The optical and mechanical properties of InGaAs can be varied by changing the ratio of InAs and GaAs, In
1-xGa
xAs.[6] Most InGaAs devices are grown on indium phosphide (InP) substrates. In order to match the lattice constant of InP and avoid mechanical strain, In
0.53Ga
0.47As is used. This composition has an optical absorption edge at 0.75 eV, corresponding to a cut-off wavelength of λ=1.68 μm at 295 K.
By increasing the mole fraction of InAs further compared to GaAs, it is possible to extend the cut-off wavelength up to about λ=2.6 μm. In that case special measures have to be taken to avoid mechanical strain from differences in lattice constants.
GaAs is lattice-mismatched to germanium (Ge) by 0.08%. With the addition of 1.5% InAs to the alloy, In0.015Ga0.985As becomes latticed-matched to the Ge substrate, reducing stress in subsequent deposition of GaAs.
Electronic and optical properties
InGaAs has a lattice parameter that increases linearly with the concentration of InAs in the alloy.[7] The liquid-solid phase diagram[3] shows that during solidification from a solution containing GaAs and InAs, GaAs is taken up at a much higher rate than InAs, depleting the solution of GaAs. During growth from solution, the composition of first material to solidify is rich in GaAs while the last material to solidify is richer in InAs. This feature has been exploited to produce ingots of InGaAs with graded composition along the length of the ingot. However, the strain introduced by the changing lattice constant causes the ingot to be polycrystalline and limits the characterization to a few parameters, such as bandgap and lattice constant with uncertainty due to the continuous compositional grading in these samples.

Properties of single crystal GaInAs
Single crystal GaInAs
Single crystal epitaxial films of GaInAs can be deposited on a single crystal substrate of III-V semiconductor having a lattice parameter close to that of the specific gallium indium arsenide alloy to be synthesized. Three substrates can be used: GaAs, InAs and InP. A good match between the lattice constants of the film and substrate is required to maintain single crystal properties and this limitation permits small variations in composition on the order of a few percent. Therefore, the properties of epitaxial films of GaInAs alloys grown on GaAs are very similar to GaAs and those grown on InAs are very similar to InAs, because lattice mismatch strain does not generally permit significant deviation of the composition from the pure binary substrate.
Ga
0.47In
0.53As is the alloy whose lattice parameter matches that of InP at 295 K. GaInAs lattice-matched to InP is a semiconductor with properties quite different from GaAs, InAs or InP. It has an energy band gap of 0.75 eV, an electron effective mass of 0.041 and an electron mobility close to 10,000 cm2·V−1·s−1 at room temperature, all of which are more favorable for many electronic and photonic device applications when compared to GaAs, InP or even Si.[1] Measurements of the band gap and electron mobility of single-crystal GaInAs were first published by Takeda and co-workers.[9]
| Property | Value at 295 K | Reference |
|---|---|---|
| Lattice Parameter | 5.869 Å | [4] |
| Band Gap | 0.75 eV | [9] |
| Electron effective mass | 0.041 | [10] |
| Light-hole effective mass | 0.051 | [11] |
| Electron mobility | 10,000 cm2·V−1·s−1 | [12] |
| Hole mobility | 250 cm2·V−1·s−1 | [12] |
FCC lattice parameter
Like most materials, the lattice parameter of GaInAs is a function of temperature. The measured coefficient of thermal expansion [13] is 5.66×10−6 K−1. This is significantly larger than the coefficient for InP which is 4.56×10−6 K−1. A film that is exactly lattice-matched to InP at room temperature is typically grown at 650 °C with a lattice mismatch of +6.5×10−4. Such a film has a mole fraction of GaAs = 0.47. To obtain lattice matching at the growth temperature, it is necessary to increase the GaAs mole fraction to 0.48.
Bandgap energy
The bandgap energy of GaInAs can be determined from the peak in the photoluminescence spectrum, provided that the total impurity and defect concentration is less than 5×1016 cm−3. The bandgap energy depends on temperature and increases as the temperature decreases, as can be seen in Fig. 3 for both n-type and p-type samples. The bandgap energy at room temperature is 0.75 eV and lies between that of Ge and Si. By coincidence the bandgap of GaInAs is perfectly placed for photodetector and laser applications for the long-wavelength transmission window, (the C-band and L-band) for fiber-optic communications.
Effective mass
The electron effective mass of GaInAs m*/m° = 0.041 [10] is the smallest for any semiconductor material with an energy bandgap greater than 0.5 eV. The effective mass is determined from the curvature of the energy-momentum relationship: stronger curvature translates into lower effective mass and a larger radius of delocalization. In practical terms, a low effective mass leads directly to high carrier mobility, favoring higher speed of transport and current carrying capacity. A lower carrier effective mass also favors increased tunneling current, a direct result of delocalization.
The valence band has two types of charge carriers: light holes: m*/m° = 0.051 [11] and heavy holes: m*/m° = 0.2.[14] The electrical and optical properties of the valence band are dominated by the heavy holes, because the density of these states is much greater than that for light holes. This is also reflected in the mobility of holes at 295 K, which is a factor of 40 lower than that for electrons.
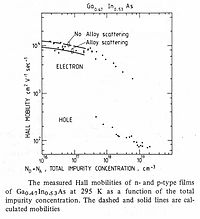
Mobility of electrons and holes
Electron mobility and hole mobility are key parameters for design and performance of electronic devices. Takeda and co-workers were the first to measure electron mobility in epitaxial films of InGaAs on InP substrates.[9] Measured carrier mobilities for electrons and holes are shown in Figure 4.
The mobility of carriers in Ga
0.47In
0.53As is unusual in two regards:
- The very high value of electron mobility
- The unusually large ratio of electron to hole mobility.
The room temperature electron mobility for reasonably pure samples of Ga
0.47In
0.53As approaches 10×103 cm2·V−1·s−1, which is the largest of any technologically important semiconductor, although significantly less than that for graphene.
The mobility is proportional to the carrier conductivity. As mobility increases, so does the current-carrying capacity of transistors. A higher mobility shortens the response time of photodetectors. A larger mobility reduces series resistance, and this improves device efficiency and reduces noise and power consumption.
The minority carrier diffusion constant is directly proportional to carrier mobility. The room temperature diffusion constant for electrons at 250 cm2·s−1 is significantly larger than that of Si, GaAs, Ge or InP, and determines the ultra-fast response of Ga
0.47In
0.53As photodetectors.
The ratio of electron to hole mobility is the largest of currently-used semiconductors.
Applications

Photodetectors
The principal application of GaInAs is as an infrared detector. The spectral response of a GaInAs photodiode is shown in Figure 5. GaInAs photodiodes are the preferred choice in the wavelength range of 1.1 μm < λ < 1.7 μm. For example, compared to photodiodes made from Ge, GaInAs photodiodes have faster time response, higher quantum efficiency and lower dark current for the same sensor area.[16] GaInAs photodiodes were invented in 1977 by Pearsall.[17]
Avalanche photodiodes offer the advantage of additional gain at the expense of response time. These devices are especially useful for detection of single photons in applications such as quantum key distribution where response time is not critical. Avalanche photodetectors require a special structure to reduce reverse leakage current due to tunnelling. The first practical avalanche photodiodes were designed and demonstrated in 1979.[18]
In 1980, Pearsall developed a photodiode design that exploits the uniquely short diffusion time of high mobility of electrons in GaInAs, leading to an ultrafast response time.[19][20] This structure was further developed and subsequently named the UTC, or uni-travelling carrier photodiode.[21] In 1989, Wey and co-workers[22] designed and demonstrated a p-i-n GaInAs/InP photodiodes with a response time shorter than 5 picoseconds for a detector surface measuring 5 μm x 5 μm.
Other important innovations include the integrated photodiode – FET receiver[23] and the engineering of GaInAs focal-plane arrays.[24]
Lasers
Semiconductor lasers are an important application for GaInAs, following photodetectors. GaInAs can be used as a laser medium. Devices have been constructed that operate at wavelengths of 905 nm, 980 nm, 1060 nm, and 1300 nm. InGaAs quantum dots on GaAs have also been studied as lasers.[25] GaInAs/InAlAs quantum-well lasers can be tuned to operate at the λ = 1500 nm low-loss, low-dispersion window for optical fiber telecommunications [26] In 1994, GaInAs/AlInAs quantum wells were used by Jérôme Faist and co-workers [27] who invented and demonstrated a new kind of semiconductor laser based on photon emission by an electron making an optical transition between subbands in the quantum well. They showed that the photon emission regions can be cascaded in series, creating the quantum cascade laser (QCL). The energy of photon emission is a fraction of the bandgap energy. For example, GaInAs/AlInAs QCL operates at room temperature in the wavelength range 3 μm < λ < 8 μm. The wavelength can be changed by modifying the width of the GaInAs quantum well.[28] These lasers are widely used for chemical sensing and pollution control.
Photovoltaics and transistors
GaInAs is used in triple-junction photovoltaics and also for thermophotovoltaic power generation.[29]
In
0.015Ga
0.985As can be used as an intermediate band-gap junction in multi-junction photovoltaic cells with a perfect lattice match to Ge. The perfect lattice match to Ge reduces defect density, improving cell efficiency.[citation needed]
HEMT devices using InGaAs channels are one of the fastest types of transistor[30][citation needed]
In 2012 MIT researchers announced the smallest transistor ever built from a material other than silicon.[31] The Metal oxide semiconductor field-effect transistor (MOSFET) is 22 nanometers long. This is a promising accomplishment, but more work is needed to show that the reduced size results in improved electronic performance relative to that of silicon or GaAs-based transistors.
펜 주립대 연구진은 2014년 잉가아스 등 복합반도체로 만든 나노와이어를 시험하기 위해 고안된 새로운 기기 프로토타입을 개발했다.[32] 이 장치의 목적은 복합 재료가 FinFET 장치 구성에서 나노 크기의 치수에서 우수한 이동성을 유지할 수 있는지를 확인하는 것이었다. 같은 연구팀이 InGaAs로 만든 트랜지스터에 대해 더 많은 연구를 실시한 결과, 낮은 공급 전압에서 온전류가 기존 실리콘 소자에 비해 매우 우수한 성능을 보인 것으로 나타났다.
2015년 2월 인텔은 2017년 7나노미터 CMOS 공정에 InGaAs를 사용할 수 있다고 밝혔다.[33]
안전 및 독성
GaInAs의 합성은, GaAs의 것과 마찬가지로, 대부분의 경우, 극도로 독성이 강한 가스인 아르신(AsH
3)의 사용을 포함한다. InP의 합성도 마찬가지로 인산(PH
3)을 가장 많이 포함한다. 이러한 기체를 흡입하면 혈류에 의한 산소 흡수가 중화되며, 독성 선량 수준이 초과될 경우 몇 분 이내에 치명적일 수 있다. 안전한 취급에는 민감한 독성 가스 감지 시스템과 자가 호흡 장치를 사용하는 것이 포함된다.[34]
일단 기질에 얇은 막으로 가인아스가 침전되면 기본적으로 불활성이며 물, 알코올, 아세톤과 같은 일반 용매에 의한 마모, 승화 또는 해체에 내성이 있다. 기기 형태에서 GaInAs의 부피는 보통 1000μm3 미만이며, 지지 기질인 InP나 GaAs의 부피와 비교해도 소홀히 할 수 있다.
국립보건원은 이러한 자료를 연구하여 다음과 같은 사실을 발견했다.[35]
- 0.01, 0.1 또는 1.0mg/m에3 피폭된 수컷 F344/N 랫드에서 갈륨 비소의 발암성 활성의 증거가 없음
- 암컷 F344/N 랫드의 발암성 활동
- 수컷 또는 암컷 B6C3F1 생쥐에서 발암성 활성이 0.1, 0.5 또는 1.0mg/m에3 노출되었다는 증거는 없다.
세계보건기구의 국제 암 연구 기구는 NIH 독성학 연구에 대한 검토를 다음과 같이 결론지었다.[36]
- 인간에게는 갈륨 비소의 발암성에 대한 불충분한 증거가 있다.
- 갈륨 비소의 발암성에 대한 실험동물들의 증거는 한정되어 있다.
- 갈륨 모이티는 암컷 쥐에서 관찰된 폐암의 원인이 될 수 있다.
REACH(화학물질의 등록, 평가, 허가 및 제한)는 제조 시 사용되거나 생산되는(쓰레기로도) 물질을 분류하고 규제하기 위한 유럽 이니셔티브다. REACH는 세 가지 독성 등급인 발암, 생식 및 돌연변이 유발 능력을 고려한다.
REACH 분류 절차는 두 개의 기본 단계로 구성된다. 1단계에서 재료가 작업장소 또는 소비자에 의해 어떻게 사용되거나 부딪힐 수 있는지에 대한 고려 없이 재료 고유의 위험을 결정한다. 2단계에서는 노출을 완화할 수 있는 절차와 함께 유해 노출 위험을 고려한다. GaAs와 InP 모두 1단계 평가 중이다. 주된 노출 위험은 연마 및 연마 시 미세한 크기의 GaAs 및 InP 입자가 발생하는 기질 준비 중에 발생한다. 웨이퍼 다이싱에서도 개별 기기를 만드는 데 유사한 우려가 적용된다. 이 입자 먼지는 호흡이나 섭취에 의해 흡수될 수 있다. 그러한 입자의 부피 대비 표면적 비율이 증가하면 화학 반응성이 증가한다.
독성학 연구는 쥐와 쥐 실험을 기반으로 한다. 액체 슬러리에서 GaAs 또는 InP 먼지를 섭취하는 효과를 테스트하는 연구는 비교할 수 없다.
REACH 절차는 예방 원칙에 따라 작용하며, "탄생성에 대한 불충분한 증거"를 "발암가능성"으로 해석한다. 그 결과, 유럽 화학청은 2010년에 InP를 발암물질과 생식독소로 분류했다.[37]
- 지침 67/548/EEC에 따른 분류 및 라벨 표시
- 분류: 카. 고양이 2; R45
- 캣 3; R62 의원
ECHA는 2010년에 GaAs를 발암물질과 생식독소로 분류했다.
- 지침 67/548/EEC에 따른 분류 및 라벨 표시:
- 분류3:카크. 고양이 1호; R45
- 고양이 2호 R60
참고 항목
참조
- ^ a b Pearsall, T. (1980). "Ga0.47In0.53As: A ternary semiconductor for photodetector applications". IEEE Journal of Quantum Electronics. Institute of Electrical and Electronics Engineers (IEEE). 16 (7): 709–720. doi:10.1109/jqe.1980.1070557. ISSN 0018-9197.
- ^ "International Union of Pure and Applied Chemistry: Home". IUPAC. Retrieved 2013-09-22.
- ^ a b Pearsall, T. P.; Hopson, R. W. (1977). "Growth and characterization of lattice‐matched epitaxial films of GaxIn1−xAs/InP by liquid‐phase epitaxy". Journal of Applied Physics. AIP Publishing. 48 (10): 4407–4409. doi:10.1063/1.323399. ISSN 0021-8979.
- ^ a b Pearsall, T. P.; Bisaro, R.; Ansel, R.; Merenda, P. (1978-04-15). "The growth of GaxIn1−xAs on (100) InP by liquid‐phase epitaxy". Applied Physics Letters. AIP Publishing. 32 (8): 497–499. doi:10.1063/1.90100. ISSN 0003-6951.
- ^ Hirtz, J.P.; Larivain, J.P.; Duchemin, J.P.; Pearsall, T.P.; Bonnet, M. (1980). "Growth of Ga0.47In0.53As on InP by low-pressure m.o. c.v.d.". Electronics Letters. Institution of Engineering and Technology (IET). 16 (11): 415–416. doi:10.1049/el:19800290. ISSN 0013-5194.
- ^ "Technology: What is InGaAs?". Sensorsinc.com. Retrieved 2013-12-02.
- ^ John W. Wagner. "Preparation and Properties of Bulk In1 − x Ga x As Alloys : SOLID STATE SCIENCE - Technical Papers". Jes.ecsdl.org. Retrieved 2013-12-02.
- ^ Pearsall, T. P.; Eaves, L.; Portal, J. C. (1983). "Photoluminescence and impurity concentration in GaxIn1−xAsyP1−y alloys lattice-matched to InP". Journal of Applied Physics. 54 (2): 1037. Bibcode:1983JAP....54.1037P. doi:10.1063/1.332122.
- ^ a b c Y. 다케다, A. 사사키, Y. 이마무라, T. 타카기, Appl의 J., 「InP 기질에 있어서의 InGaAs의
0.53
0.47 전자 이동성과 에너지 격차」. 물리학 47, 5405-7 (198), https://doi.org/10.1063/1.322570 - ^ a b Nicholas, R. J.; Portal, J. C.; Houlbert, C.; Perrier, P.; Pearsall, T. P. (1979-04-15). "An experimental determination of the effective masses for GaxIn1−xAsyP1−y alloys grown on InP". Applied Physics Letters. AIP Publishing. 34 (8): 492–494. doi:10.1063/1.90860. ISSN 0003-6951.
- ^ a b Hermann, Claudine; Pearsall, Thomas P. (1981-03-15). "Optical pumping and the valence‐band light‐hole effective mass in GaxIn1−xAsyP1−y (y≃2.2x)". Applied Physics Letters. AIP Publishing. 38 (6): 450–452. doi:10.1063/1.92393. ISSN 0003-6951.
- ^ a b c Pearsall, T.P.; Hirtz, J.P. (1981). "The carrier mobilities in Ga0.47In0.53 as grown by organo-mettalic [sic] CVD and liquid-phase epitaxy". Journal of Crystal Growth. Elsevier BV. 54 (1): 127–131. doi:10.1016/0022-0248(81)90258-x. ISSN 0022-0248.
- ^ Bisaro, R.; Merenda, P.; Pearsall, T. P. (1979). "The thermal‐expansion parameters of some GaxIn1−xAsyP1−x alloys". Applied Physics Letters. AIP Publishing. 34 (1): 100–102. doi:10.1063/1.90575. ISSN 0003-6951.
- ^ Lin, S. Y. (1989). "Cyclotron resonance of two-dimensional holes in strained-layer quantum well structure of (100)In0.20Ga0.80As/GaAs" (PDF). Applied Physics Letters. 55 (7): 666–668. Bibcode:1989ApPhL..55..666L. doi:10.1063/1.101816.
- ^ T.P. Pearsall, "InGaAs Photodetectors" in Properties of Lattice-Matched and Strained Indium Gallium Arsenide, ed P.Bhattacharya, (London, IEE Press, 1993) pp267-77.
- ^ Pearsall, T.P.; Pollack, M.A. (3 June 1985). Tsang, W. T. (ed.). Photodiodes for Optical Fiber Communication. SEMICONDUCTORS AND SEMIMETALS. 17. Academic Press. pp. 174–246. ISBN 978-0-08-086417-4.
- ^ T.P. Pearsall and R.W. Hopson, Jr, Electronic Materials Conference, Cornell University, 1977, published in J. Electron. Mat. 7, pp.133-146, (1978)
- ^ Nishida, Katsuhiko (1979). "InGaAsP heterostructure avalanche photodiodes with high avalanche gain". Applied Physics Letters. 35 (3): 251–253. Bibcode:1979ApPhL..35..251N. doi:10.1063/1.91089.
- ^ Pearsall, T. (1981). "A Ga0.47In0.53As/InP heterophotodiode with reduced dark current". IEEE Journal of Quantum Electronics. 17 (2): 255–259. Bibcode:1981IJQE...17..255P. doi:10.1109/JQE.1981.1071057.
- ^ Pearsall, T.P.; Logan, R.A.; Bethea, C.G. (1983). "GaInAs/InP large bandwidth (> 2 GHz) PIN detectors". Electronics Letters. Institution of Engineering and Technology (IET). 19 (16): 611–612. doi:10.1049/el:19830416. ISSN 0013-5194.
- ^ Shimizu, N. (1998). "InP-InGaAs uni-traveling-carrier photodiode with improved 3-dB bandwidth of over 150 GHz". IEEE Photonics Technology Letters. 10 (3): 412–414. Bibcode:1998IPTL...10..412S. doi:10.1109/68.661427.
- ^ Wey, Y. G.; Crawford, D. L.; Giboney, K.; Bowers, J. E.; Rodwell, M. J.; Silvestre, P.; Hafich, M. J.; Robinson, G. Y. (1991-05-13). "Ultrafast graded double‐heterostructure GaInAs/InP photodiode". Applied Physics Letters. AIP Publishing. 58 (19): 2156–2158. doi:10.1063/1.104991. ISSN 0003-6951.
- ^ Veteran, J.L. (1982). "Schottky barrier measurements on p-type In0.53Ga0.47As". Thin Solid Films. 97 (2): 187–190. Bibcode:1982TSF....97..187V. doi:10.1016/0040-6090(82)90227-9.
- ^ "Sensors Unlimited - InGaAs Near and Short Wave Infrared (SWIR) Cameras, Arrays, and Photodiodes". Sensorsinc.com. Retrieved 2013-09-22.
- ^ Bimberg, D.; Kirstaedter, N.; Ledentsov, N.N.; Alferov, Zh.I.; Kop'ev, P.S.; Ustinov, V.M. (1997). "InGaAs-GaAs quantum-dot lasers". IEEE Journal of Selected Topics in Quantum Electronics. Institute of Electrical and Electronics Engineers (IEEE). 3 (2): 196–205. doi:10.1109/2944.605656. ISSN 1077-260X.
- ^ K. Alavi, H. Temkin, A.Y. Cho, and T.P. Pearsall, "AlInAs-GaInAs Multi Quantum-Well Lasers Emitting at 1.55μm", Appl. Phys. Lett. 4244, 845-847 (1983)
- ^ Faist, J.; Capasso, F.; Sivco, D. L.; Sirtori, C.; Hutchinson, A. L.; Cho, A. Y. (1994-04-22). "Quantum Cascade Laser". Science. American Association for the Advancement of Science (AAAS). 264 (5158): 553–556. doi:10.1126/science.264.5158.553. ISSN 0036-8075.
- ^ J. Faist, Quantum Cascade Laser, (Oxford, Oxford University Press, 2013)
- ^ M.Tan, L.Ji, Y.Wu, P.Dai, Q.Wang, K.Li, T.Yu, Y.Yu, S.Lu and H.Yang, "Investigation of InGaAs thermophotovoltaic cells under blackbody radiation", Applied Physics Express 7,p. 096601 (2014), https://doi.org/10.7567/APEX.7.096601
- ^ [1] Archived January 4, 2006, at the Wayback Machine
- ^ "Tiny compound semiconductor transistor could challenge silicon's dominance".
- ^ Thathachary, Arun V.; Agrawal, Nidhi; Liu, Lu; Datta, Suman (January 1, 2014). "Electron Transport in Multigate InxGa1–x As Nanowire FETs: From Diffusive to Ballistic Regimes at Room Temperature". Nano Letters. 14 (2): 626–633. Bibcode:2014NanoL..14..626T. doi:10.1021/nl4038399. PMID 24382089.
- ^ Sebastian Anthony (23 Feb 2015). "Intel forges ahead to 10nm, will move away from silicon at 7nm". Ars Technica. Retrieved 28 Nov 2019.
- ^ The environment, health and safety aspects of indium gallium arsenide sources (such as trimethylgallium, trimethylindium and arsine) and industrial hygiene monitoring studies of standard MOVPE have been reviewed. Shenai-Khatkhate, D.V.; et al. (2004). "Environment, health and safety issues for sources used in MOVPE growth of compound semiconductors". Journal of Crystal Growth. 272 (1–4): 816–821. Bibcode:2004JCrGr.272..816S. doi:10.1016/j.jcrysgro.2004.09.007.
- ^ "NTP Technical Report on the Toxicology and Carcinogenesis Studies of Gallium Arsenide" (PDF). Ntp.niehs.nih.gov. Retrieved 2013-09-22.
- ^ "IARC Monographs on the Evaluation of Carcinogenic Risks to Humans" (PDF). Monographs.iarc.fr. Retrieved 2013-09-22.
- ^ "Homepage - ECHA". Echa.europa.eu. Retrieved 2013-09-22.
External links
- NSM data archive at the Ioffe Institute, St. Petersburg, Russia




